How to Lower Alkalinity in a Pool
There is more to lowering alkalinity than just pouring acid in the pool.
To lower alkalinity correctly, use diluted acid to reduce alkalinity by no more than 20 ppm per day. Alkalinity should be reduced in a gradual, methodical way. Do not just pour in a large dose of acid and hope for the best. You will tank the pH so low that there will be serious consequences to the pool surface and equipment.
Textbooks recommend total alkalinity between 80-120 ppm (mg/L). Orenda's recommendations depend on the LSI as a whole, as well as your primary chlorine type. If you have a saltwater pool or a liquid chlorine pool, you can have less than 80 ppm TA if you have enough calcium hardness to offset it. We consider over 120 ppm TA high, and over 150 ppm very high. We have seen tap water over 300 ppm in some places in the country, and that is extraordinary.
Lowering alkalinity to get to a manageable level is important, but so is lowering it in a controlled way, as to not drop the pH too much and cause damage to the pool and equipment.
How does acid lower alkalinity?
Acid neutralizes alkali in the water by adding hydrogen (H+) to it. For bicarbonate and carbonate alkalinity–the majority of the alkalinity species in swimming pools–acid converts these ions into carbonic acid, which is dissolved CO2.
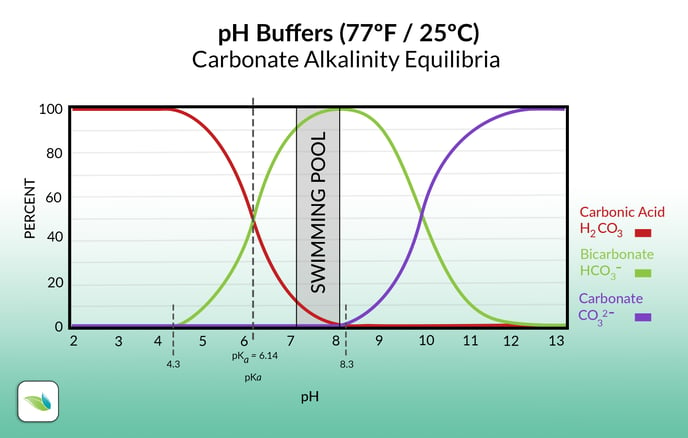
As the pH changes, the percentage of each species of carbonate alkalinity changes. Most pool chemistry is dominated by Bicarbonate (HCO3-) ions, as shown in the graph above.
When you add acid to the pool, Hydrogen ions are added to alkalinity, converting it into carbonic acid (H2CO3). Carbonic acid brings the pH of the pool down, and is technically not considered alkalinity. Carbonic acid cannot accept any more Hydrogens, therefore is no longer a buffer against pH reduction.
Other types of alkalinity that contribute to Total Alkalinity
Total Alkalinity is mostly carbonate alkalinity, but it also includes cyanurate alkalinity, and if you use borate, that contributes too. Both need to be subtracted from total alkalinity when doing the LSI formula (which calls for the carbonate alkalinity only). But don't worry about the math, the Orenda Calculator™ does all of these adjustments for you.
Alkalinity is a buffer against pH reduction because it neutralizes acid. So again, to reduce alkalinity, you must neutralize alkali with acid. But how?
How to correctly lower alkalinity in a pool
Dilute, dilute, dilute, dilute. The key to effective alkalinity reduction is the opposite of what conventional wisdom in the pool industry has claimed for years: the "column pour". That's a myth, and it's a destructive myth at that. The truth is, no matter how you add acid to the water, the same amount of alkali will be neutralized.
We at Orenda know that the density of muriatic acid is 1.18x that of water, which looks like this when you add food coloring to it:
Lower a maximum of 20 ppm at a time. Ideally 10-15 ppm or less. Take your time when lowering alkalinity! It's not a race. Gradually bring it down with diluted acid, so that the acid has time to spread around and neutralize alkali before it sinks to the bottom of the pool and begins to attack the surface at the bottom (plaster/cement, vinyl liner, fiberglass, etc.).
Patience and discipline are how you do this right. Do not rush it.
Related Orenda articles and procedures: