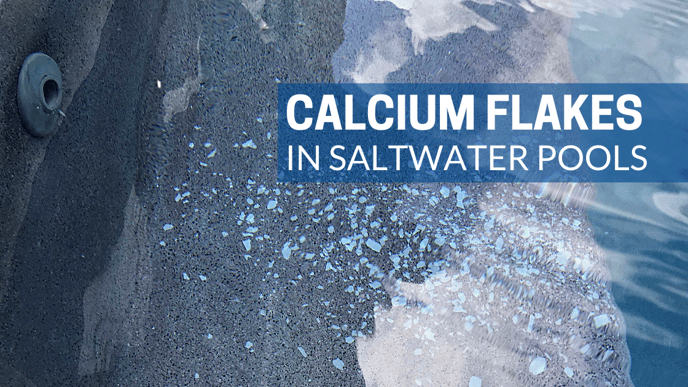
If you have a saltwater pool, you may have white calcium flakes in it. Here's why.
Calcium flakes are a prevalent problem in saltwater pools. Flakes are caused by carbonate scale formation in the salt cell. Essentially what happens is the salt system is doing its thing, creating chlorine using electrolysis. The byproduct of chlorine generation is a very high pH substance called Sodium Hydroxide (NaOH) on the cathode sides of the salt cell blades. (As an aside, this sodium hydroxide is eventually neutralized by the low pH of the chlorine gas, and is not actually the cause of the pH rising in the pool.)
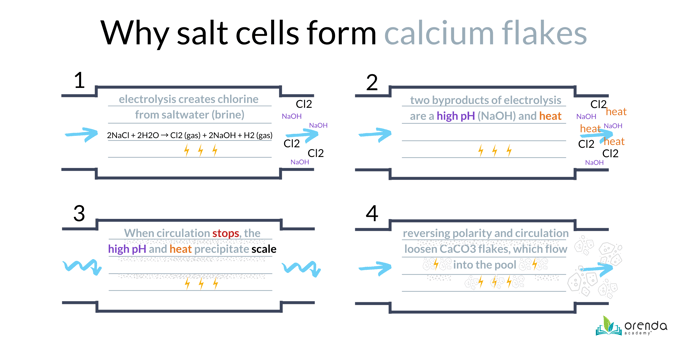
This ultra-high pH causes a localized LSI violation, which forms some carbonate scale on the blade. It should be noted that while the water is circulating, there is usually not enough time for scale to actually form, because water passes through the cell in fractions of a second. But once circulation slows down or stops, more scale is likely to form unless the system cools down and water flushes the sodium hydroxide out of the cell.

Photo: Inside a salt cell that has scale formation
As the salt cell reverses polarity (alternates electrical current direction), the scale fractures into flakes, which are carried into the pool and usually land near the return inlets. Typically these are found in a spa first, if you have a pool/spa combination.
What are the flakes made of?
In our experience, white flakes in salt pools are calcium carbonate. There are sources online that claim they are calcium phosphate, but we have seen no evidence of that. We are not sure where that came from, but if you had calcium phosphate scale on the salt cell, it would not be flaking off into your pool...it's much more difficult to remove than that.
If you want to do an experiment to prove (or disprove) if they are calcium carbonate, simply collect some flakes and put them in a small plastic container with some diluted muriatic acid. If they fizz and disappear, they were calcium carbonate. Spoiler alert: they are.
How can calcium flakes be prevented?
Prevention takes the following steps, in order of importance:
- Balance the LSI of your water. On a saltwater pool, when the water temperature is warm enough for the salt system to be producing chlorine, understand that the pH will naturally rise, thanks to Henry's Law of physics, and the loss of CO2 created during the electrolysis process thanks to Hydrogen gas bubbles (H2) creating turbulence in the cell. Knowing this, it is imperative that you limit how high the pH can go by adjusting your carbonate alkalinity. Here's a chart to illustrate the pH ceiling, and how it is dependent on your carbonate alkalinity and water temperature:
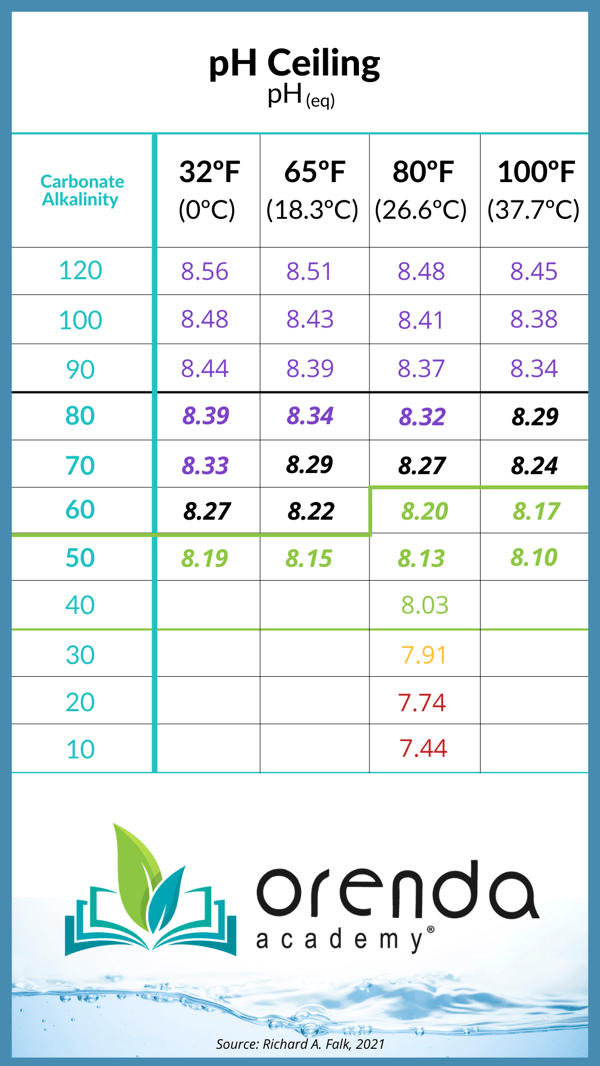
Learn more about how to contain pH here. We are not saying you need very low alkalinity, but we are saying you need to be aware of your carbonate alkalinity (TA - [CYA x ~0.33] = Carbonate alkalinity). Generally we have found salt pools have much more success with about 60 ppm TA, and those pools need higher calcium hardness to compensate for the lower TA on the LSI.
On the Orenda Calculator, if you select show on the secondary readings toggle, you will see the carbonate alkalinity AND the pH ceiling. Both numbers are adjusted in real-time based on your chemistry.
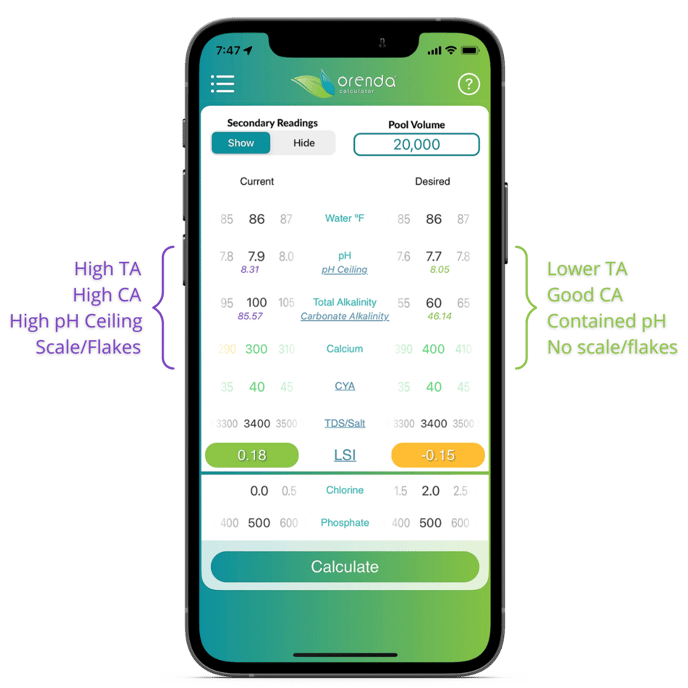
This strategy allows you to maintain LSI balance not only today, but throughout the week until your next treatment on the pool. Assume pH will naturally rise (because it will unless you have an auto-cover suppressing pH or an acid feeder), and embrace it. The pH ceiling is the highest the pH can go naturally, so be sure the Orenda App shows you a green LSI value at 8.1 pH.
- Next, find a way to flush the salt system (after it turns off) with circulating water for a few minutes before the pump shuts off. This could be as simple as programming the pump to dial down to its slowest speed, triggering the salt system to shut off, yet water is still flowing through the salt system. It could also be accomplished by programming a delay on the controller (if you have one) to signal the salt system to shut off a few minutes before the pump. We're not engineers here, we're just saying the salt cell needs to be flushed out and cooled down, or else the flakes will likely continue.
- Finally, if you're still struggling with calcium flakes, you can chelate the calcium in your water using SC-1000. It may not solve the problem on its own, but SC-1000 has proven to be very effective against flakes when the LSI is balanced.