PR-10,000 clouded my water but did not reduce my tested phosphate levels. Why?
PR-10,000 reacts with all types of phosphates, but does the test kit?
There is a difference between testing for phosphates and removing phosphates with PR-10,000. There are different types of phosphates, yet phosphate test kits generally only measure one type: orthophosphate. PR-10,000, on the other hand, removes any and all types of phosphate.
If phosphates are present in your water, one of our brand guarantees is that PR-10,000 will react with and remove them, according to how you dosed the water. But there are rare instances using PR-10,000 where:
-
- Phosphate levels stay about the same, or
- Phosphate levels climb
But how can that be?! The answer has to do with the type of phosphates that PR-10,000 is reacting with.
Did PR-10,000 cloud your water?
If PR-10,000 clouds the water, phosphates are being removed from solution. You're watching it happen before your eyes. The clouding is caused by PR-10,000 reacting with the phosphate and converting it into a solid. In your pool, these solids come out of solution as a cloud of fine white dust.

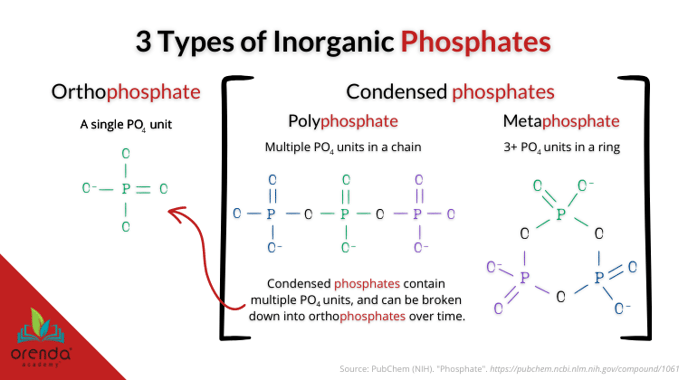
Types of phosphates
But which phosphates? There are different types of phosphates that do not show up on most phosphate test kits.
- Organic phosphates (not common in swimming pools)
- Inorganic phosphates
- Condensed phosphates
- Polyphosphate (chain of linked orthophosphates)
- Pyrophosphate
- Metaphosphate (ring of linked orthophosphates)
- Phosphoric acid(s)
- Phosphonic acid(s)
- Orthophosphate
- Condensed phosphates
Those are only a few variations of phosphates. Here's a list of even more. The point is, test kits generally do not measure condensed phosphates in water. They measure orthophosphates only.
So you could actually have far higher levels of phosphates than you tested in your water, and therefore you might not see a noticeable reduction in phosphate after using PR-10,000. Put simply, you are likely removing other forms of phosphate, and are not seeing a noticeable reduction in orthophosphate testing.
Converting to Orthophosphate
Another factor to consider is many condensed phosphates will convert into orthophosphate over time. In the presence of sunlight and an oxidizer like chlorine, these phosphate forms break down into orthophosphates. We know this to be true not only from research, but from field experiments we have done ourselves.
Condensed inorganic phosphates such as polyphosphate and metaphosphate are built upon multiple units of orthophosphate linked together. See the diagram above.
Conclusion
If PR-10,000 clouds up your water, you are absolutely reducing phosphates. They just might not be in the form of orthophosphates (yet). PR-10,000 does not care. It wipes out any form of phosphate in the water, and a cloud of white dust is the byproduct.
