What do hydration and carbonation mean?
Hydration and carbonation are part of cement mixing and curing. This article explains the difference between them.
Cement chemistry involves converting various calcium compounds into others that harden over time. This involves different chemical processes, but most people refer to the entire process as "curing". But we should be more specific because there are two stages of curing.
Hydration
Hydration occurs immediately when water mixes with cement to create new compounds. Namely, water activates the cement to convert it from a dry powder into a pourable slurry which will eventually re-harden into concrete. Once hydration begins, the clock starts ticking, because within a few hours the cement will harden into concrete.
While water hydrating cement creates many compounds, the compound we talk about most in swimming pool plaster is calcium hydroxide, partly because it takes at least 30-60 days to carbonate.
A secondary process occurs after hydration, which could be considered "dehydration", though it is not called that. Essentially, moisture leaves concrete, which can shrink its volume, leading to shrinkage cracks. That's why it is so important to keep concrete wet while curing.
Carbonation
Carbonation is the process of converting calcium hydroxide into calcium carbonate. Carbonation begins to occur when carbon dioxide in the air touches the freshly applied cement as the pool is filling up. But most carbonation happens when the cement is submerged in water, and carbonate alkalinity (bicarbonate and carbonate ions) combine with calcium hydroxide.
Both bicarbonate (HCO3-) and carbonate ions (CO3--) contain CO3, which of course contains CO2 (carbon dioxide). Here's a simplified equation showing what carbonation looks like:
Ca(OH)2 + CO2 → CaCO3 + H2O
calcium hydroxide + carbon dioxide → calcium carbonate + water
Swimming Pool Plaster
The interaction between water and curing cement (plaster) is important to understand. If the water is hungry for calcium carbonate saturation (low on the LSI), it will find the most soluble form of calcium available and dissolve it into solution. The most soluble form of calcium in a curing cement surface is calcium hydroxide.
We want carbonation to occur within the cement. But when the water steals calcium hydroxide, it gets carbonated outside the cement, and turns into white calcium carbonate dust. This is called plaster dust.
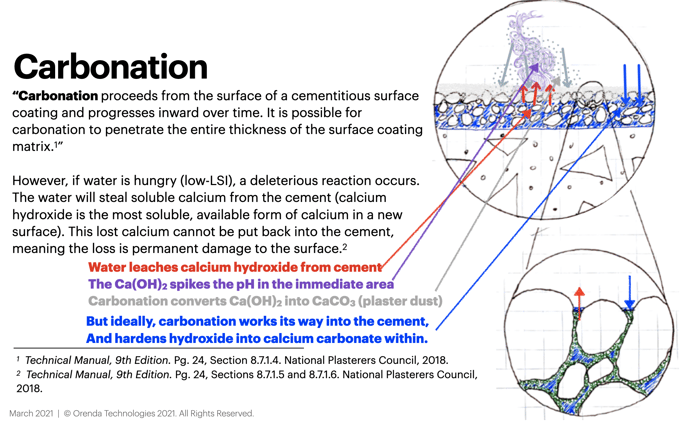
If the water is LSI balanced, however, carbonation can occur as it is supposed to: penetrating carbonates into the cement from the water to convert calcium hydroxide into calcium carbonate. This reinforces the strength of the cement. This is the final stage of "curing", as most of us would think of it.
Conclusion
Hydration is when water mixes with cement, beginning the process of chemical conversion into hardened concrete. For swimming pool plaster, hydration occurs on the mixing truck.
Carbonation is when carbonates or carbon dioxide interact with calcium hydroxide to convert it into calcium carbonate. Ideally this occurs within the cement.
If low-LSI pool water interacts with this cement, it can steal calcium hydroxide for itself, which leads to carbonation occurring outside of the cement. The result is calcium carbonate dust that lands on the surface called plaster dust.
Related: