What is bromine?
Bromine is a halogen element, like chlorine, that is used as a disinfectant in water. It's the most popular alternative to chlorine in spas.
Bromine is a chlorine alternative mostly used in spas and hot tubs. It is a halogen element located just beneath chlorine on the Periodic Table of Elements. This means it is less reactive than chlorine, but in the right application, it is still an effective sanitizer and oxidizer. However, bromine chemistry differs from chlorine in a few key ways.
How bromine works
There are three bromine products used in spas and pools: BCDMH, DBDMH, and Sodium bromide. We go into specifics about these products in our article on bromine, but just know that they all put bromide ions (Br-) in the water. Bromide ions can be oxidized into the active killing form of bromine, Hypobromous acid (HOBr). HOBr is bromine's equivalent to chlorine's HOCl.
The main difference between chlorine and bromine is that bromide ions can be oxidized back into active HOBr indefinitely. It's a cycle.
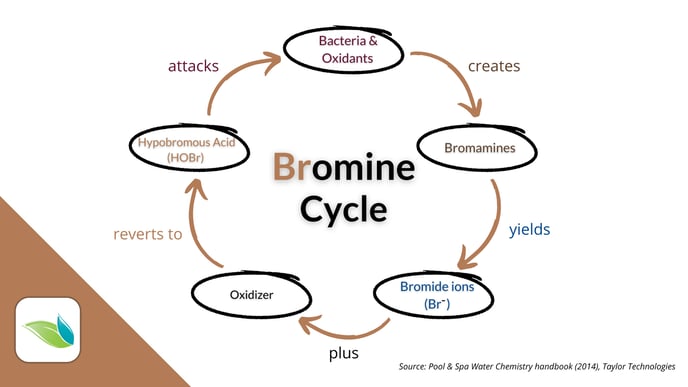
It takes a salt system's electrolysis to recharge inert Chloride ions (Cl-) in a chlorine pool. But in a bromine pool, insert bromide ions (Br-) can be oxidized by various oxidizers: chlorine, potassium monopersulfate, ozone or hydroxyl radicals. That said, ozone, AOP and even UV light (from direct sunlight or a UV system) create harmful byproducts called bromates.
Bromates are created a number of ways:
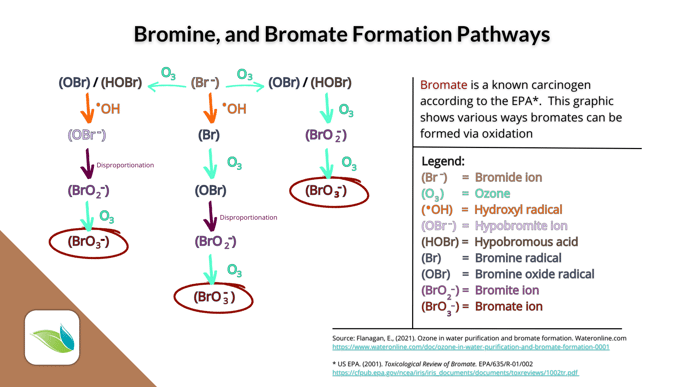
As long as bromide ions are in the water, oxidizers will convert them into active HOBr. This can be a real problem for chlorine pools using sodium bromide as an algaecide.
Bromine byproducts
Bromine oxidizes and kills things, then gets reduced eventually down to bromide ions (Br-). If the HOBr oxidizes nitrogen compounds, bromamines and other disinfection byproducts (DBPs) will be created.
Perhaps the most important of these DBPs are called trihalomethanes (THMs). In short, trihalomethanes are harmful and we do not want them in water. There are four types of trihalomethanes that are possible in bromine pools and spas, as compared to only one in a chlorine pool.
-1.jpg?width=688&height=387&name=trihalomethanes%20(THM)-1.jpg)
Is chlorine compatible with bromine?
Chlorine can be used to oxidize bromide ions back into hypobromous acid (HOBr). But bromine should not be used in chlorine pools. All bromine products leave bromide ions in the water that will become a source of chlorine demand. In fact, when the amount of bromide ions exceeds the chlorine in the pool, the chlorine pool effectively becomes a bromine pool. And there is no turning back until you get the bromides out of the water.
You can think of bromides like chlorides, or even nitrates. There are no practical ways to remove bromides other than draining/diluting them out or reverse osmosis filtration.
So when comparing chlorine vs. bromine, chlorine is the preferred sanitizer.
Where can bromine be used?
Because of the formation of bromates, most pools and spas should not use bromine. The only applications where bromine is appropriate would be indoor pools or spas that have no secondary systems like UV, ozone or AOP. That being said, bromine is more expensive to operate because of the need for a secondary oxidizer to recharge it, so you may find it cost prohibitive to use in large bodies of water.
Most of the bromine sold in our industry is for covered hot tubs. And if the hot tub is exposed to direct sunlight, it should be covered at all times when not being used. Avoid daytime use to avoid bromate formation.