Why does Alkalinity rise in a pool using a CO2 feeder for pH control?
CO2 reduces pH but not alkalinity. Alkalinity tends to rise in CO2-fed pools. Here's why.
Alkalinity rises due to the excess hydroxides (lye) from non-stabilized chlorine that would otherwise be negated when using acid.
Acid reduces both pH and alkalinity (by converting bicarbonate into carbonic acid), so the excess hydroxide (OH-) from liquid chlorine or cal hypo is negated. But when using CO2, CO2 does nothing to these excess hydroxides, so they accumulate. Eventually, those hydroxides build up enough to create a noticeable increase in total alkalinity.
pH is largely determined by the amount of carbon dioxide (CO2) in your water
The more CO2 dissolved in your water, the lower the pH. This is why injecting CO2 lowers pH. Some of the dissolved CO2 converts into carbonic acid (H2CO3), which lowers the pH. The opposite is also true. The loss of CO2 raises the pH. This is why aeration raises the pH.
↑ CO2 = ↓ pH
↓ CO2 = ↑ pH
Acid, on the other hand, creates carbonic acid by converting bicarbonate (HCO3-), thus reducing the amount of bicarbonate (and therefore lowering total alkalinity).
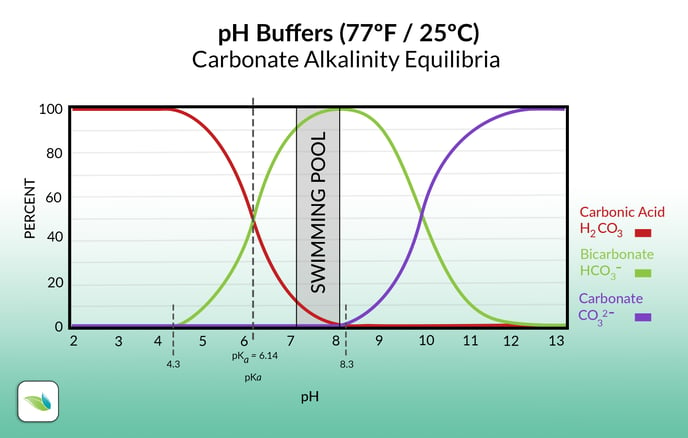
Learn more about these equilibria here.