Can I maintain high alkalinity (>120 ppm) and low calcium hardness (<200 ppm) if it keeps my LSI balanced?
Some pools have fill water that is very high in total alkalinity, and it makes it difficult to maintain LSI balance.
First of all, it is possible to have LSI balance with high total alkalinity (TA) and low calcium hardness (CH). But it's unlikely to stay balanced for long, due to TA driving the pH up. But sometimes, thanks to tap water chemistry, those are the cards you are dealt, and you need to find a way to address high TA out of the tap.
If your tap water chemistry is not favorable to maintaining LSI balance, you are not alone. Large regions of the United States face this scenario, whether it be low LSI or high LSI out of the tap. Most areas have low-LSI tap water, but in some cases, tap water can have very high alkalinity, which in turn drives the pH up too. In this case, we recommend working your alkalinity down to a favorable level that allows you to consistently maintain LSI balance.
High TA leads to high pH. Together, they lead to high LSI
First of all, it's entirely possible to have LSI balance with alkalinity over 120 ppm. We have seen tap water with 200+ ppm TA, and yet the LSI is balanced because of lower calcium hardness levels. The problem, however, is that the pH ceiling will be much higher than you want it to be because of the high alkalinity. See the pH ceiling chart below:
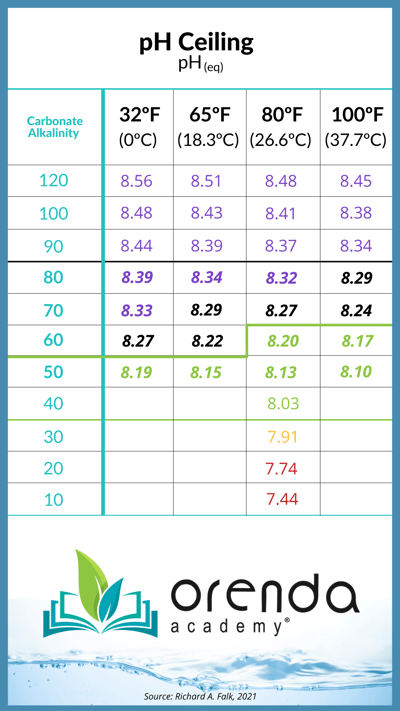
From strictly an LSI perspective, high TA is not necessarily a bad thing. But when we factor in where the pH is going to naturally rise to (the pH ceiling), you're virtually guaranteed to have a high-LSI violation by the end of the week. Scale and carbonate clouding are likely to happen. We prefer to avoid such circumstances.
Get TA down and CH up to better manage LSI
We recommend working your alkalinity down in a gradual, methodical way. No more than 20 ppm at a time with diluted acid. If needed, when you're adding this diluted acid, you can pre-dissolve calcium chloride in the bucket too, which will raise calcium hardness (CH). This will offset the reduction in LSI when you reduce TA.
Assuming you use the Orenda app and select values that keep the LSI balanced as you lower TA, your TA and CH will offset each other and the LSI can remain balanced the entire time. This is how we are able to reduce TA without risking etching or other surface damage.
↓TA with acid + ↑CH with calcium chloride = neutral LSI impact
Worth the chemical costs?
One of the questions we get is whether or not it's worth reducing the TA and raising the CH. Indeed, it costs money to buy all that acid and calcium chloride.
But think about the costs of trying to keep the LSI balanced if you don't. If you leave your TA high (over 120 ppm, in this example), your acid consumption will be more every time you treat the pool for two reasons:
- The pH will rise higher and faster, and
- The amount of acid required to do the same pH correction is proportional to the TA. The higher the TA, the more acid required.
So, in our opinion, you will save money in the long run by getting your TA down and CH up, and getting your CH:TA ratio (minimum 3:1, recommended 4+:1) in order.