My tap water tested zero phosphates, but my pool has phosphates. Why?
Phosphates can get into water in many different ways, including pool chemicals, the environment around the pool, and the source water.
If you have high phosphates in your pool, they had to come from somewhere. There are three primary sources of phosphates:
- Pool chemicals (namely sequestering agents/scale inhibitors)
- The environment around the pool
- Tap water
To further complicate things, most phosphate test kits only test orthophosphate (PO4-). But there are many different types of phosphates found in pools. It's common to test much lower levels of phosphates than are actually in the water. We expand on this in another help article, here.
Usually, high phosphates come from the use of phosphate-based sequestering agents.
Phosphate-based pool chemicals
Most metal and scale inhibitor products are phosphate-based. These include phosphonates, polyphosphates, metaphosphates, phosphoric acid and phosphonic acid. These variants are called condensed phosphates because they are either in a molecular chain or ring formation.
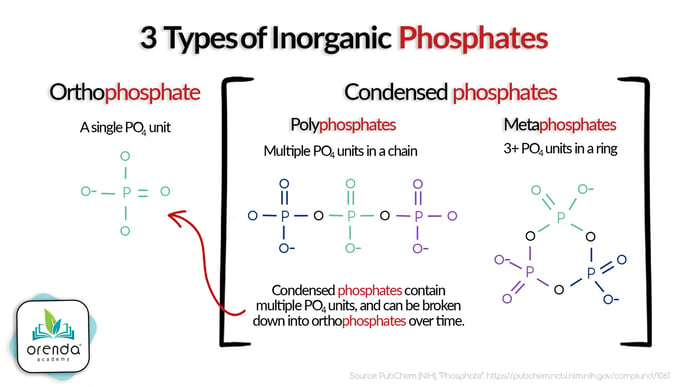
Condensed phosphates often will not show up on a phosphate test, because the test is only showing their building block, an orthophosphate (PO4-).
These chemicals are highly effective for sequestering metals and assisting in metal removal via a metal filter. They do their job and they are widely available in any pool store. They have a place in pool care if there are heavy metals in the water, or persistent scaling issues. Users just need to be aware that they will also be putting phosphates in the water.
It should be noted that our chelating agent SC-1000 does NOT contain phosphates.
Why don't condensed phosphates show up on a phosphate test?
Since we do not manufacture test kits, it's not our place to explain exactly how these tests work. But we do know through field experience (orders of magnitude more field experience than our own, thanks to customer feedback) that it is common for sequestering agents to not be recognized by phosphate tests for several days, or even weeks.
Our understanding is that these compounds will eventually break down into measurable orthophosphates over time, thanks to direct sunlight and oxidation (we're told). According to at least one study, the combination of UV light and chlorination is effective at breaking down the phosphonate sequestering agent 1-Hydroxyethane-1, 1-diphosphonic acid (HEDP).1
Phosphates from the environment around the pool
Phosphates are an essential part of the living world. Plants, soils, and other organic materials that may be around the pool contain phosphates. When wind and rain bring these things into the pool, phosphates come with them.
Bathers also introduce phosphates, though the concentration can vary wildly, depending on how clean the person is. Dirty hands and feet from playing in the grass will bring in more phosphates than someone fresh out of the shower.
Fertilizer products that may be in the lawn (or sprayed onto plants) are loaded with phosphorus too.

In some more interesting examples, wildfire smoke, ash and soot contain phosphates, just like Saharan dust that can travel across the Atlantic.
The point is, phosphates are everywhere in the world around us. They are bound to get into pools one way or another.
Phosphates in drinking water
Finally, there is tap water. Yes, this article answers the question about phosphates not in tap water, but let's be honest...most people don't test their tap water unless there's a noticeable issue. We believe testing tap water is important.
Decades ago, phosphates were present primarily in well water, where the water naturally dissolved it while percolating through the soils into the water table below the ground. Nowadays, however, almost all municipalities across the nation deliberately treat drinking water with phosphates. This occured largely due to an EPA directive following the Flint, Michigan water crisis in 2014.
To make a long story short, the EPA rules have shifted behavior in the water treatment world. Notably, phosphate-based products are widely used now. Particularly phosphate-based sequestering agents, just like the ones mentioned in earlier in this article.
Closing
Phosphates come from all over the place, and they complicate water chemistry. They are not urgent to remove from the pool, especially if you have enough free chlorine relative to cyanuric acid (FC:CYA). Generally, best practice is to keep phosphate levels below 500 ppb (Pillar 3).
So no matter how the phosphates are getting in the water, PR-10,000 can find them and remove them. Consider the red cap test if your phosphate test is inconclusive.
1 Nan Huang, Wen-Long Wang, Zi-Bin Xu, Qian-Yuan Wu, Hong-Ying Hu. (2019). UV/chlorine oxidation of the phosphonate antiscalant 1-Hydroxyethane-1, 1-diphosphonic acid (HEDP) used for reverse osmosis processes: Organic phosphorus removal and scale inhibition properties changes. The Journal of Environmental Management. Vol. 237, pp. 180-186.