Sanitize vs. Disinfect vs. Oxidize | The Roles of Chlorine in Swimming Pools
Chlorine has many jobs in swimming pools. Let's define them.
In its strong, killing form, Hypochlorous Acid (HOCl), chlorine's main job is to keep swimming pool water clean and safe. But that's not its only job.
There are two primary responsibilities of chlorine in pools:
- Kill microorganisms, and
- Oxidize non-living contaminants (metals, nitrogen compounds, and organics).
There are two levels of "kill" that chlorine can accomplish: sanitization, and disinfection. There's a higher level called sterilization, but that's not applicable in swimming pools.
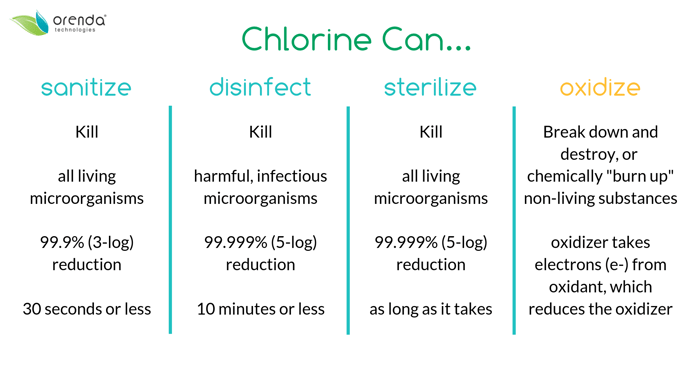
HOCl will easily sanitize and disinfect if there's enough of it available. But with a heavy oxidant demand and/or too much CYA in the water, chlorine's killing speed is slowed down.
Chlorine the Killer
Without a doubt, chlorine's primary job is to kill things. And it's exceptionally good at it. A very small amount of Hypochlorous acid (HOCl) can kill bacteria and most viruses in just seconds. And since killing strength is actually about killing speed, we need to bring up a metric called Contact Time (CT).
CT values are used in health codes and standards to determine how much chlorine is needed in certain conditions. This is how free chlorine minimums are determined. We encourage you to do the research if you want to find out the exact CT values of certain diseases like E-Coli and Giardia. You will find that in the right conditions, chlorine is very effective at killing these things.
The only question then is the thoroughness of the kill. And that's where we find the difference between sanitizing and disinfecting.
Sanitization
Sanitization is a 3-log reduction within 30 seconds or less. A three log reduction means killing 99.9% of microorganisms. Pretty darn thorough, right? Except there's a much higher level, two more logarithms of thoroughness.
Technically, sanitization is for any living microorganism. This includes plants like algae.
Disinfection
Disinfection is a 5-log reduction within 10 minutes or less. Usually, the same amount of chlorine that will sanitize is enough to disinfect too. While sanitization is about killing any living microorganisms, disinfection is only for infectious microorganisms (germs, viruses, parasites, etc.).
Since most of the sanitizer demand in swimming pools is reproducing algae, most people just use the term sanitization (or 'sanitation', which is technically incorrect but used interchangeably in the pool industry).
Chlorine the Oxidizer
While chlorine is excellent at killing germs and algae, the bulk of its workload in a pool is actually non-living oxidants, which we call the oxidant demand.
Related: Non-living Organics and Oxidants | Pillar 2
It is estimated that well over 90% of chlorine demand is oxidation. And there are three types of oxidants in pools that chlorine addresses:
- Metals (iron, copper, manganese, etc.)
- Nitrogen compounds (ammonia, urea, nitrates, etc.)
- Non-living Organics
- Natural organics (tannins, body oils, pet dander, bird droppings, mucus, etc.)
- Synthetic organics (deodorant, lotions, hair products, cosmetics, sunscreen, etc.)
The vast majority of the oxidant demand are non-living organics. Generally speaking, the more complex the molecule, the more chlorine it takes to break down. There are some exceptions to this, but it's a reasonable way of thinking about things.
For instance, it takes more chlorine to break down sunscreen than it does to oxidize iron. Iron oxidation is almost instantaneous, whereas sunscreen may take days and reduce far more chlorine.
Summary
Chlorine's primary job is to kill microorganisms to keep water clean and safe. Living microorganisms are referred to as the sanitizer demand. But that's not the majority of chlorine's responsibilities. Compared to the non-living oxidant demand, the sanitizer demand is a small percentage of chlorine's job. And the majority of the oxidant demand are non-living organics and oils.
To optimize chlorine efficiency, supplement chlorine against the oxidant demand so it has enough in residual to kill what it needs to kill.