What are borates? And why are they in the Orenda Calculator?
Borates affect pool chemistry and impact the LSI. We included them in the calculator because of their LSI impact.
Borates are forms of boron used in various products like laundry detergent boosters and cleaning agents. They are used in swimming pools for their pH buffering capabilities.
We explain borates in much more detail in our blog.
Borates impact the LSI because they contribute to Total Alkalinity (TA)
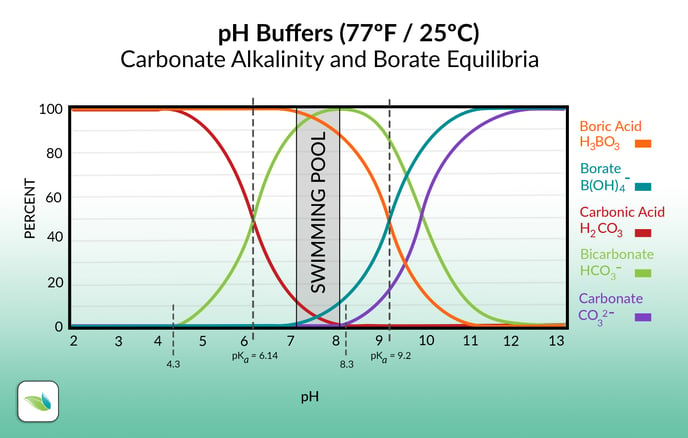
Much like carbonic acid and bicarbonate alkalinity, boric acid and borate are in equilibrium. Based on the pH of the water, the percentage of each species is shown on the graph above. The exact pH where the species intersect (50-50%) is where the pH buffering capacity is strongest. For carbonic acid and bicarbonate, that's 6.14 pH. For boric acid and borate, it's 9.2 pH.
These pH's are called pKa values. The closer the pH gets to the pKa value, the more resistance to change in pH it will face.
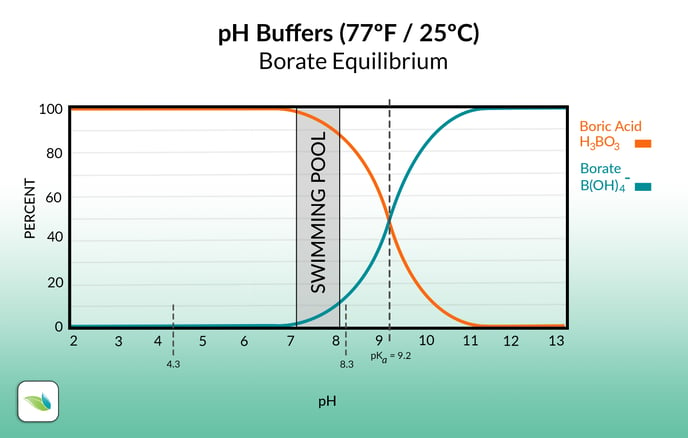
Contributing to TA means borates need to be deducted from TA to calculate Carbonate Alkalinity, which is needed for calculating the LSI
We included borates in the Orenda Calculator™ because of its LSI impact. The more borates you have, the lower the carbonate alkalinity. You can see this by opting to "show" secondary readings.
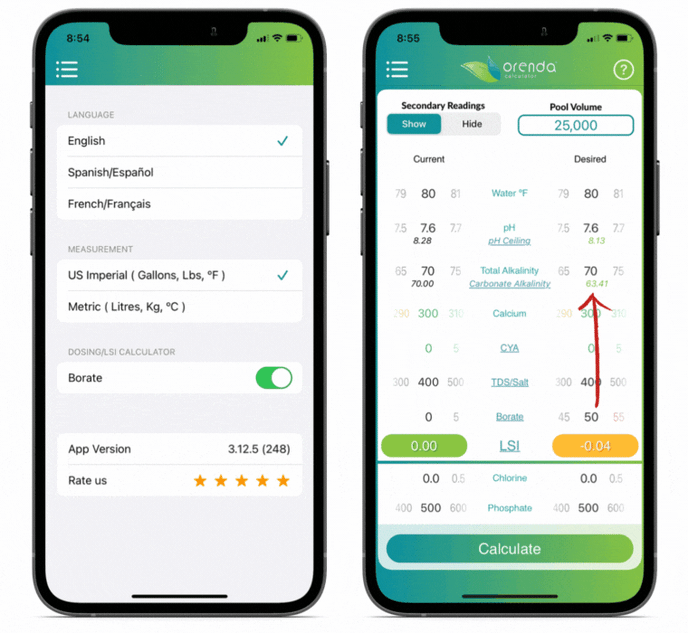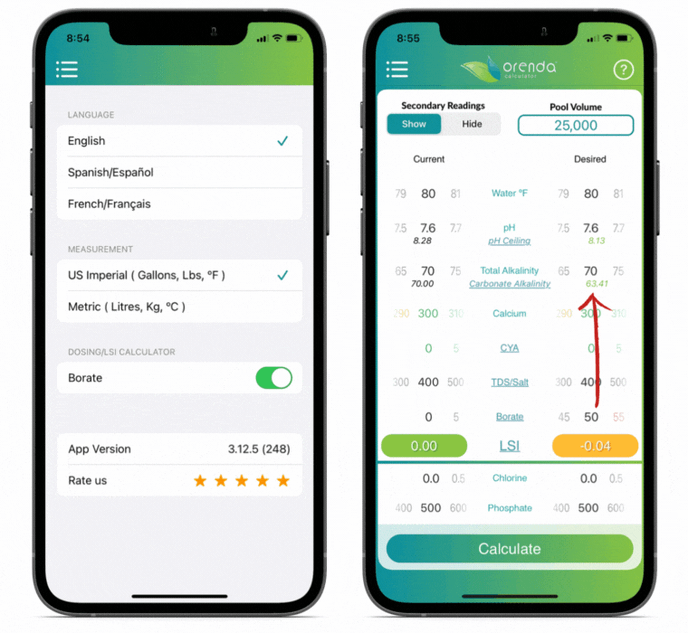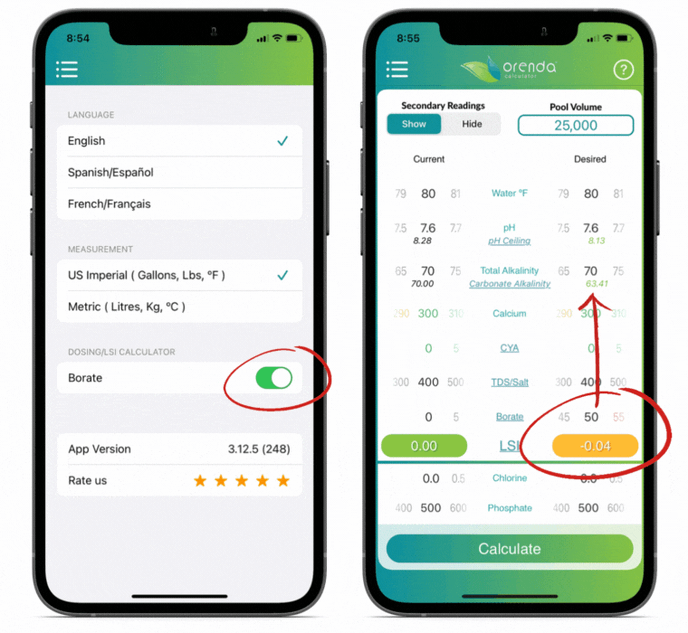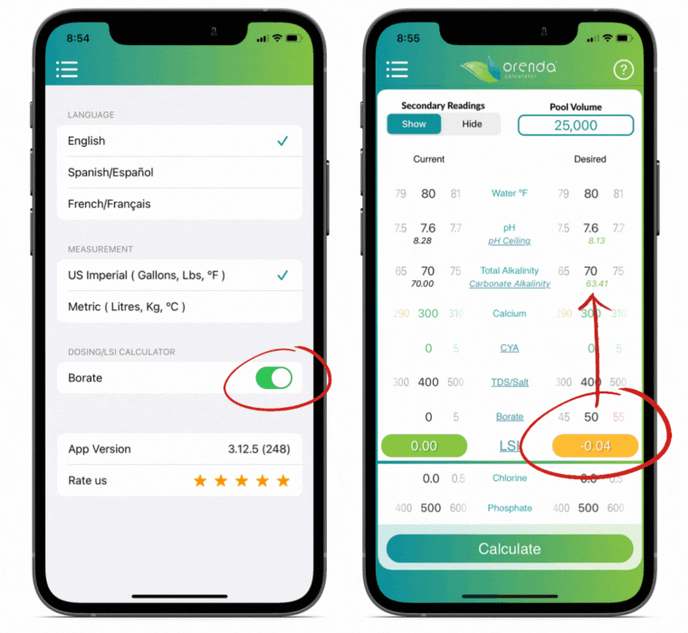
Orenda does not endorse the use of borates, but if you use them, do not exceed 50 ppm. And remember to factor them into your LSI using the Orenda Calculator™.