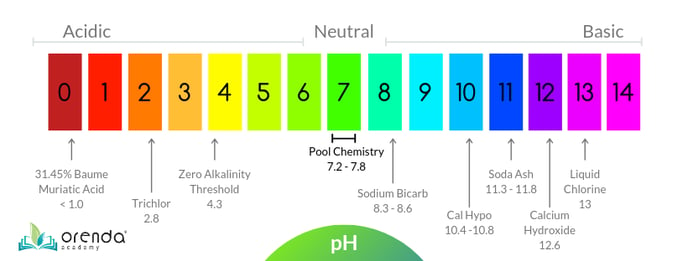
What is pH?
pH tells us how acidic or basic a substance is on a logarithmic scale from 0-14, where 7.0 is perfectly neutral.
pH stands for "potenz Hydrogen", or in English, the "power of Hydrogen."
pH can be defined in many ways, included below. pH...
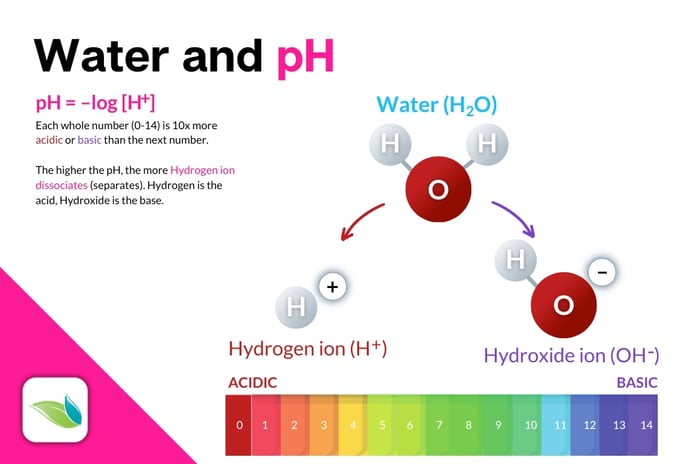
- is the negative logarithm of Hydrogen ions: pH = -log[H+].
- is based on a water molecule (H2O) splitting into Hydrogen ion (H+), the acid, and Hydroxide ion (OH-), the base.
- is a value between 0 and 14, which determines how acidic or basic a substance is. 7.0 (in the middle) is perfectly neutral. Every whole number is 10x greater or less than the next whole number. (Example: 6.0 pH is 10x more acidic than 7.0. 5.0 pH is 100x more acidic than 7.0, because it's 10x10.
- determines how connected or dissociated a Hydrogen ion is to various substances, such as carbonate alkalinity and free chlorine (without CYA in the water).
- impacts just about every other aspect of water chemistry
The nitty gritty of pH
It is technically the negative logarithm of Hydrogen ions.
pH = -log[H+]
To simplify this, think about a water molecule, H2O. When Hydrogen dissociates from H2O, you're left with a free Hydrogen ion (H+) and a Hydroxide ion (OH-).
H2O ⇌ (H+) + (OH−)
Each whole number on the 0-14 pH scale is 10x greater or less than the numbers around it. So 6 is 10x more acidic than 7, and 12 is 10x more basic than 11. This scale is logarithmic, meaning those 10's compound on each other. So 5 is 100x more acidic than 7 (10x10=100), and 13 is one million times more basic than 7.
How does pH impact water chemistry?
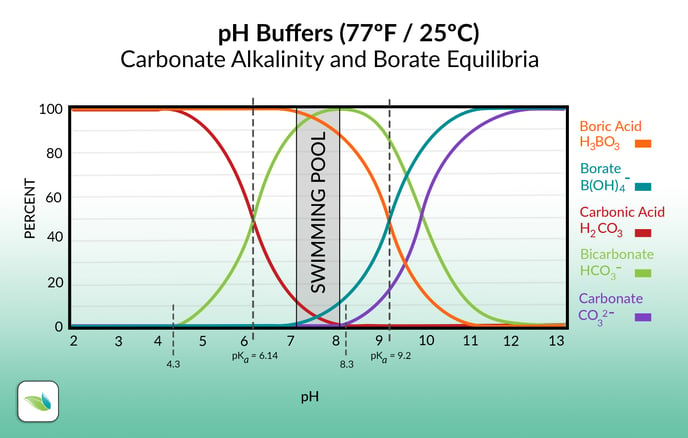
Basically, everything in pool chemistry is impacted by pH in some way or another. It's all about Hydrogen concentration. So the higher the pH, the less likely Hydrogen is to stick around, and vice versa. Here's a chart showing carbonate alkalinity and borate equilibria. Notice the higher the pH goes, the less Hydrogen is attached to the alkalinity and boric acid ions:
Why pH is important, according to swimming pool industry textbooks
The conventional wisdom in the swimming pool business says pH is very important, and they cite three main reasons why:
- pH controls the strength of chlorine (%HOCl)
- pH impacts bather comfort
- pH dictates how corrosive or scale-forming water is
Let's quickly dissect these, and link to our relevant blogs that expand on these topics and more.
1. pH controls the strength of chlorine (%HOCl)
This is true when there is no Cyanuric Acid present in the water. The graphic shown in most books shows that lower pH means stronger chlorine because of the higher percentage of the strong form of chlorine, Hypochlorous Acid (HOCl).
When chlorine is added to water (in any form), eventually, there is a dissociation between Hypochlorous acid (HOCl) and its far weaker and slower counterpart, Hypochlorite Ion (OCl-). These two substances are in equilibrium with each other and are totally pH-dependent. Notice the only difference is whether or not Hydrogen is attached:
HOCl ⇌ (H+) + (OCl-)
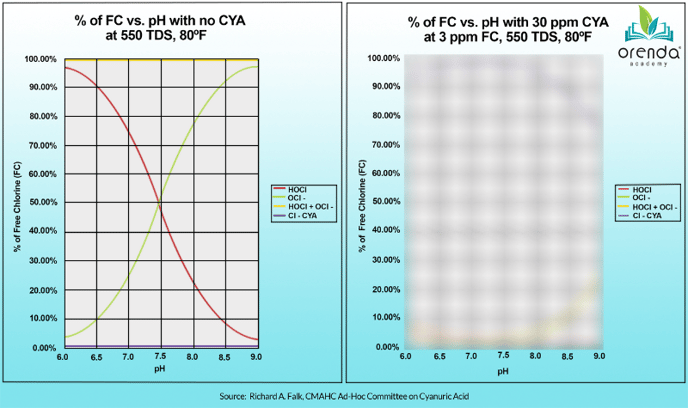
But again, this is without CYA in the water. Look at this chart from a different perspective. The chart above is recreated on the left side, while the same parameters are repeated on the right side, except with 30 ppm CYA:

Where is the red line on the right chart? You'll see that the %HOCl is below 3%, and shows very little difference between 7.0 and 8.5 pH. This is because chlorine bound to CYA is not as pH-dependent. It's totally different chemistry. More on chlorine, pH and CYA relationships here.
2. pH impacts bather comfort.
We disagree. With as much personal time in swimming pools as we have, we do not buy the argument that pH makes a dramatic impact on bather comfort. We looked into the argument about the pH of human tears, and found it to be lacking. We believe it is actually chloramines and other disinfection byproducts (DBPs) that cause bather discomfort. Eye and skin irritation is far more likely when combined chlorine levels exceed 0.2 ppm than if the pH is at 7.2 or even lower.
Think about the bottled water brands out there. Some have pH in the 5's, and others are alkaline and have a pH of 9+. That's an enormous pH difference, and yet both are safe to drink, and neither irritate our skin, mouth or stomach. Swimming pools should never have such a radical pH range, so we do not buy this argument whatsoever. Debunked.
3. pH dictates how corrosive or scale-forming water is.
Well, kind of, yes. But pH is just one of six factors that make up the Langelier Saturation Index (LSI). It's the LSI that dictates the corrosivity or scale-forming tendencies of water. So a pH being out of whack can be compensated for with other factors, like alkalinity and calcium hardness. We firmly believe in LSI balance year-round. In fact, it's our First PIllar of proactive pool care.
CO2 and pH
The amount of dissolved CO2 in water impacts the pH, and in many ways determines the pH. This is due to the carbonate alkalinity equilibrium, but that's a conversation for another day. Learn more about carbonate alkalinity here.
The more CO2 dissolved in water, the lower the pH (and vice versa):
↑CO2 = ↓pH
↓CO2 = ↑pH
Aeration raises pH because CO2 leaves the water. Algae also raises pH because it consumes CO2, and takes it out of solution.
More CO2 will reduce pH, which is why many commercial pools inject CO2 for pH control instead of acid. Acid reduces pH by converting bicarbonate alkalinity into carbonic acid (H2CO3). Carbonic acid is dissolved CO2 (H2O + CO2 ⇌ H2CO3).
The pH can be suppressed by either feeding acid, using acidic trichlor chlorine, or with a solid autocover, which prevents–or at least slows–the natural loss of CO2.
Henry's Law and the natural off-gassing of CO2
In simplified terms, Henry's Law states that any gas dissolved in a liquid must equalize with that same gas above the liquid. A carbonated drink (like soda or beer) has no bubbles until the bottle is opened. Then, you can hear CO2 escape the bottle and bubbles appear in the middle of the drink. This is Henry's Law in action. It's showing CO2 escaping the highly-pressurized drink to equalize with the lower-pressure room.
The exact same physics applies to swimming pools. Just like a soda or beer, eventually the CO2 will equalize with the air above the drink, and the drink will go flat. Pools will "go flat" too, though we never see the bubbles because the surface area is so much greater, and the percentage of CO2 is so much lower. But rest assured, CO2 is leaving the pool until it equalizes with the air above the pool.
This natural off-gassing of CO2 means the pH naturally rises until the pool "goes flat." That exact point of CO2 equilibrium is when the pH equalizes, called "pH(eq)", which we at Orenda call the pH ceiling. It tells us exactly how high the pH can naturally rise on its own. See the chart below:
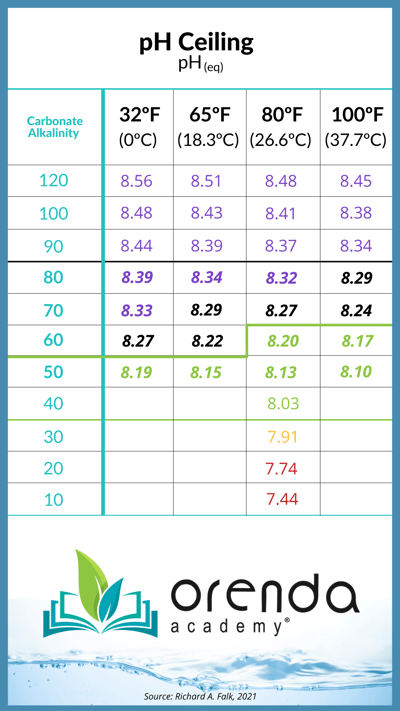 The pH cannot naturally rise above its ceiling. But it can be forced if the pool is overdosed with acid or soda ash.
The pH cannot naturally rise above its ceiling. But it can be forced if the pool is overdosed with acid or soda ash.
This allows us to predict where pH is going, and contain pH instead of trying to control it. See the links in the summary for more information.
Summary
pH tells us how acidic or basic a substance is, and it's all about the concentration of Hydrogen ions. The more Hydrogen, the more acidic. The less Hydrogen, the more alkaline (or basic).
Here are some relevant articles if you want to read further: